Page Content
Office Hours:
Announcements:
Dr. Jacqueline Houston
Office: Sequoia 420A Phone: (916) 278-2583 email: jhouston@csus.edu
Chemistry Department phone: (916) 278-6684 Fax: (916) 278-4986
Mailing Address:
CSUS Department of Chemistry
6000 J Street
Sacramento, CA 95819-6057
Courses:
Chem 110 Inorganic Chemistry
Chem 110L Advanced Inorganic Chemistry
Chem 1B General Chemistry II
Chem 6A General Chemistry for Health Science
Fall 2016 Courses :Chem 110/110L
Research in Inorganic Chemistry:
Research Synopsis:
One of the most challenging and pressing issues confronting our nation is the safe and cost-effective management of environmental pollutants and the remediation of hazardous waste sites. To capture aqueous hazardous waste, porous metal-oxides/hydroxides and silicates with diverse compositions, structures, and macroscopic morphologies have been used for decades. However, most metal-oxide materials are so structurally complex that reactions that take place at the solid-surface interface are not well understood. In order to enhance adsorption and exchange properties of metal-oxide materials, a molecular-level understanding of how the solid oxide surface reacts is critical.
Our research efforts focus on aqueous transition-metal chemistry of metal-oxo clusters. We establish fundamental relationships between structure and reactivity for metal-oxide materials by employing the use of aqueous Nb, Mo, W, and Rh-oxo model compounds (See Figure 1). We synthesize our transition metal clusters using traditional inorganic synthetic techniques and characterize our compounds using NMR (1H, 17O, 103Rh, 95Mo), electronic spectroscopy and infrared spectroscopy. Once synthesized, we measure rates of ligand substitution at the metal clusters using variable-temperature NMR spectroscopy. Understanding rates of substitution are particularly important since these reactions are similar to those that take place at metal-oxide remediation materials.
Most Recent Papers:
Kris A. Pineda, James C. Fettinger, Jacqueline R. Houston "Synthesis, structure, and substitution reactivity of a new bi-oxo capped molybdenum cluster: [Mo3(u3-O)2(u2-O2CCH2Cl)(H2O)2(OH)]+" Inorganic Chemistry Communications 48 (2014) 90-93.
Jacqueline R. Houston and Andrew J. Burton "Solvent dependent mechanistic pathways for n-O2CCH3 from the Mo3(u3-O)2 anion" Inorganica Chimica Acta 407 (2013) 210-215.
Kris A. Pineda, James C. Fettinger, Jacqueline R. Houston " A new aqueous molybdenum cluster with a Mo3(u3-CCH3)(u3-O) trinuclear core" Inorganica Chimica Acta 392 (2012) 485–489.
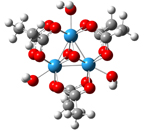
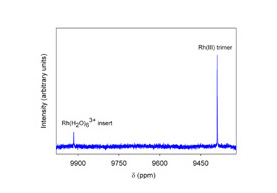
Figure 1. A typical metal-oxo cluster is shown on the left. Bound aqua ligands (η-OH2) and bridging oxos (μ3-O) are common structural features of metal-oxide/hydroxide surfaces. Shown on the right is a 103Rh NMR spectrum of a trinucleaur rhodium cluster.
Our Research Group
Science Olympiad (2015)

Nick after positively identifying one unknown sample.

Rich and John trying to Help...

Nick Williams

John Hayes

Graduate student Rich Brookins
Office: Sequoia 420A Phone: (916) 278-2583 email: jhouston@csus.edu
Chemistry Department phone: (916) 278-6684 Fax: (916) 278-4986
Mailing Address:
CSUS Department of Chemistry
6000 J Street
Sacramento, CA 95819-6057
Courses:
Chem 110 Inorganic Chemistry
Chem 110L Advanced Inorganic Chemistry
Chem 1B General Chemistry II
Chem 6A General Chemistry for Health Science
Fall 2016 Courses :Chem 110/110L
Research in Inorganic Chemistry:
Research Synopsis:
One of the most challenging and pressing issues confronting our nation is the safe and cost-effective management of environmental pollutants and the remediation of hazardous waste sites. To capture aqueous hazardous waste, porous metal-oxides/hydroxides and silicates with diverse compositions, structures, and macroscopic morphologies have been used for decades. However, most metal-oxide materials are so structurally complex that reactions that take place at the solid-surface interface are not well understood. In order to enhance adsorption and exchange properties of metal-oxide materials, a molecular-level understanding of how the solid oxide surface reacts is critical.
Our research efforts focus on aqueous transition-metal chemistry of metal-oxo clusters. We establish fundamental relationships between structure and reactivity for metal-oxide materials by employing the use of aqueous Nb, Mo, W, and Rh-oxo model compounds (See Figure 1). We synthesize our transition metal clusters using traditional inorganic synthetic techniques and characterize our compounds using NMR (1H, 17O, 103Rh, 95Mo), electronic spectroscopy and infrared spectroscopy. Once synthesized, we measure rates of ligand substitution at the metal clusters using variable-temperature NMR spectroscopy. Understanding rates of substitution are particularly important since these reactions are similar to those that take place at metal-oxide remediation materials.
Most Recent Papers:
Kris A. Pineda, James C. Fettinger, Jacqueline R. Houston "Synthesis, structure, and substitution reactivity of a new bi-oxo capped molybdenum cluster: [Mo3(u3-O)2(u2-O2CCH2Cl)(H2O)2(OH)]+" Inorganic Chemistry Communications 48 (2014) 90-93.
Jacqueline R. Houston and Andrew J. Burton "Solvent dependent mechanistic pathways for n-O2CCH3 from the Mo3(u3-O)2 anion" Inorganica Chimica Acta 407 (2013) 210-215.
Kris A. Pineda, James C. Fettinger, Jacqueline R. Houston " A new aqueous molybdenum cluster with a Mo3(u3-CCH3)(u3-O) trinuclear core" Inorganica Chimica Acta 392 (2012) 485–489.


Figure 1. A typical metal-oxo cluster is shown on the left. Bound aqua ligands (η-OH2) and bridging oxos (μ3-O) are common structural features of metal-oxide/hydroxide surfaces. Shown on the right is a 103Rh NMR spectrum of a trinucleaur rhodium cluster.
Our Research Group
Science Olympiad (2015)
Nick after positively identifying one unknown sample.
Rich and John trying to Help...

Nick Williams

John Hayes

Graduate student Rich Brookins
Office Hours:
Th 10am-12pm
Announcements:

