Support Page Content
Human Subjects Research
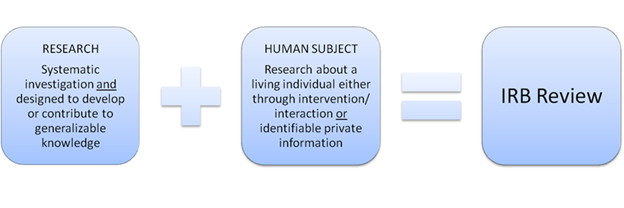
The Institutional Review Board (IRB) reviews human subjects research conducted by Sacramento State researchers to ensure that participants are treated ethically and protected from harm.
1. IRB Submission Determination
Please use this self-determination tool or review our SharePoint guidance to determine whether your activity requires IRB submission and review. We welcome questions early to avoid unnecessary submissions.
IRB Submission Self-Determination Tool
Not Human Subjects Research Guidance (SharePoint)
2. CITI Training
Human Subjects Research certification must be current upon IRB application submission.
CITI Auto-Enroll Links (New!)
Select only one option below. The links below will automatically enroll you in your selected Human Subjects Research course. It will show under Courses Ready to Begin:
All Undergraduate and Masters Students
Biomedical Faculty, Staff, Doctoral Student
Social-Behavioral-Educational Faculty, Staff, Doctoral Student
3. IRB Submissions in Cayuse
a. Submit a New IRB Application
Follow the Start and Submit a New Study guide for step-by-step instructions on creating your Cayuse application.
Cayuse IRB Library (SharePoint)
b. Prepare Your Protocol
The IRB provides topic-specific guidance to support you in developing a compliant, participant-centered protocol. Resources include templates and examples for:
- Informed consent forms
- Demographic questionnaires
- Incentive best practices
- Data security and storage best practices
- And more
Research Protocol Guidance (SharePoint)
c. Manage Your Approved Study
Once your study is approved, you can manage it through Cayuse:
- Modifications: Request changes to your approved protocol.
- Incident Reports: Report unanticipated problems or adverse events.
- Renewals: Submit continuing reviews for ongoing Full Board studies.
- Study Closure: Close your protocol when research activities are complete.
4. Review Timelines
Deadlines do not apply to the majority of research. Please review our normal timeline and plan accordingly.
Other Situations
a. Classroom Projects
Students required to complete a research project as part of a course requirement (except for theses/dissertations) within the semester are fully under the supervision of their professor and do not require IRB review. Faculty are encouraged to use the Student Form, when research is about human subjects, and require students to submit these forms for faculty review prior to research commencing. The absence of IRB review does not mean an absence of research ethics.
Student Form for Classroom Project (SharePoint)
b. Reliance Agreements for Cooperative Research
Reliance Agreements should only be considered when a Sacramento State affiliate will be working with an external investigator to conduct research. These agreements should only be entered when both campuses are:
- Collecting informed consent,
- Collecting data (identifiable or not), and/or
- Analyzing identifiable data.
This agreement involves the cooperation of both campuses, and administrators of both IRBs must be included in the communication and routing of this form.
Reliance Agreements (SharePoint)
c. External Investigators
If you are not affiliated with Sacramento State and would like to enroll participants from our campus, please email your IRB approval letter and approved informed consent form(s).
Contact IRB
Still have questions?